GE HealthCare announces FDA approval of Flyrcado (flurpiridaz F 18) injection PET radiotracer for enhanced diagnosis of coronary artery disease
- GE HealthCare’s FDA-approved flurpiridaz F 18 PET radiotracer, Flyrcado, delivers higher diagnostic efficacy in patients with known or suspected coronary artery disease (CAD), compared to SPECT MPI, the predominant procedure used in nuclear cardiology today
- With a half-life over ten times longer than currently approved cardiac PET radiotracers, Flyrcado brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD
- Available as a unit dose - or ready-to-use, Flyrcado has the potential to expand clinician and patient access to PET myocardial perfusion imaging (MPI)
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240927874133/en/
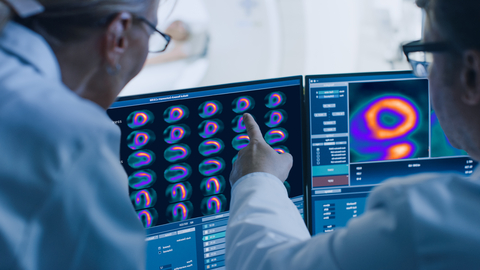
Healthcare professionals reviewing myocardial perfusion PET images. (Photo: Business Wire)
With a half-life of 109-minutes—significantly longer than existing PET MPI tracers—Flyrcado removes the need for on-site tracer production and generator maintenance and enables distribution to a wide network of hospitals and imaging centers. This longer half-life also means Flyrcado brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD, enabling the most robust protocol for evaluating ischemia in patients. Furthermore, clinicians would have the ability to rescan a patient during the same imaging session in the event of technical difficulties, rather than rescheduling an additional scan.
Dr.
CAD is the most common form of heart disease and remains the leading cause of death for men and women in the
Dr. Mouaz Al-Mallah, MD, MSc, MASNC, immediate Past President of the
Kevin O’Neill, CEO of the
Flyrcado is one of three F 18 imaging agents in GE HealthCare’s portfolio of FDA-approved molecular imaging PET products, joining radiopharmaceutical PET imaging agents Cerianna™ (fluoroestradiol F 18) injection, used to detect estrogen receptor positive lesions as an adjunct to biopsy in patients with metastatic and recurrent breast cancer, and Vizamyl™ (flutemetamol F 18) injection which is a PET tracer for imaging of the brain to estimate beta amyloid neuritic plaque density in adult patients who are being evaluated for Alzheimer’s disease or other causes of cognitive decline.
Flyrcado will be available in initial
As a leading global medical technology and pharmaceutical diagnostics innovator,
About
Follow us on LinkedIn, X , Facebook, Instagram, and Insights for the latest news, or visit our website https://www.gehealthcare.com/ for more information.
https://www.gehealthcare.com/products/molecular-imaging-agents/flyrcado
INDICATIONS AND USAGE OF FLYRCADO™:
FLYRCADO™ (flurpiridaz F 18) injection, for intravenous use important safety information
IMPORTANT SAFETY INFORMATION
Indications and Usage
FLYRCADO is a radioactive diagnostic drug indicated for positron emission tomography (PET) myocardial perfusion imaging (MPI) under rest or stress (pharmacologic or exercise) in adult patients with known or suspected coronary artery disease (CAD) to evaluate for myocardial ischemia and infarction.
Contraindications
None
Warnings and Precautions
- Risk associated with exercise or pharmacologic stress: Patients evaluated with exercise or pharmacologic stress may experience serious adverse reactions such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction, stroke, and seizure. Perform stress testing in the setting where cardiac resuscitation equipment and trained staff are readily available. When pharmacologic stress is selected as an alternative to exercise, perform the procedure in accordance with the pharmacologic stress agent’s prescribing information.
- Radiation risks: FLYRCADO contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to minimize radiation exposure to patients and health care providers. Advise patients to hydrate before and after administration and to void.
Adverse Reactions
- Most common adverse reactions occurring during FLYRCADO PET MPI under rest and stress (pharmacologic or exercise) (incidence ≥ 2%) are dyspnea, headache, angina pectoris, chest pain, fatigue, ST segment changes, flushing, nausea, abdominal pain, dizziness, and arrhythmia.
Use in Specific Populations
- Pregnancy
There are no data on use of flurpiridaz F 18 in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes based on the radiation dose from flurpiridaz F 18 and the gestational timing of exposure.
FLYRCADO contains ethanol (a maximum daily dose of 337 mg anhydrous ethanol). If considering FLYRCADO administration to a pregnant woman, inform the patient about the potential for adverse pregnancy outcomes associated with ethanol exposure during pregnancy.
- Lactation
Temporarily discontinue breastfeeding. A lactating woman should pump and discard breastmilk for at least 8 hours after FLYRCADO administration.
- Pediatric Use
Safety and effectiveness of FLYRCADO in pediatric patients have not been established.
To report SUSPECTED ADVERSE REACTIONS, contact
For full prescribing information, click here. For important safety information, please click here.
INDICATIONS AND USAGE OF CERIANNA™:
INDICATIONS AND USAGE
CERIANNA is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer.
Limitations of Use
Tissue biopsy should be used to confirm recurrence of breast cancer and to verify ER status by pathology. CERIANNA is not useful for imaging other receptors, such as human epidermal growth factor receptor 2 (HER2) and the progesterone receptor (PR).
CONTRAINDICATIONS
None.
ADVERSE REACTIONS
In Clinical Trials (n=1207) the most common adverse reactions seen occurred at a rate < 1% were injection-site pain and dysgeusia.
To report SUSPECTED ADVERSE REACTIONS, contact
For full Prescribing Information click here.
INDICATIONS AND USAGE OF VIZAMYL™:
PRODUCT INDICATIONS AND USE
VIZAMYL™ (Flutemetamol F 18) injection is indicated for positron-emission tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) or other causes of cognitive decline. A negative scan indicates sparse to no neuritic plaques, inconsistent with a diagnosis of AD at the time of image acquisition. A negative scan result reduces the likelihood that a patient’s cognitive impairment is due to AD. A positive scan indicates moderate to frequent amyloid neuritic plaques. This amount of amyloid neuritic plaque has been shown to be present in patients with AD but may also be present in patients with other neurologic conditions as well as in older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations.
Limitations: A positive scan does not establish a diagnosis of AD or other cognitive disorder. The safety and effectiveness of Vizamyl have not been established for predicting the development of dementia or other neurologic conditions or for monitoring responses to therapies.
Important Safety Information About VIZAMYL™ (Flutemetamol F 18) injection
CONTRAINDICATIONS
• Known hypersensitivity to Vizamyl or any excipient, including polysorbate 80
ADVERSE REACTIONS
• The most commonly reported adverse reactions in clinical trials were flushing (2%), increased blood pressure (2%), headache (1%), nausea and dizziness (1%)
For full prescribing information, including additional important safety information, please click here.
1 Maddahi J, Agostini D, Bateman TM, Bax JJ, Beanlands RSB, BermanDS, Dorbala S, Garcia EV, Feldman J, Heller GV, Knuuti JM,
2 Schindler, T. H., Bateman, T. M., Berman, D. S., Chareonthaitawee, P., De Blanche, L. E., Dilsizian, V., Dorbala, S., Gropler, R. J., Shaw, L., Soman, P., Winchester, D. E., Verberne, H., Ahuja, S., Beanlands, R. S., Di Carli, M. F., Murthy, V. L., Ruddy, T. D., & Schwartz, R. G. (2020). Appropriate use criteria for PET myocardial perfusion imaging.
3 Friede A, O’Carroll PW, Thralls RB, Reid JA.
4 Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: A report from the
5 Miller, R. J. H., Bednarski, B. P., Pieszko, K., Kwiecinski, J., Williams, M. C., Shanbhag, A., Liang, J. X., Huang, C., Sharir, T., Hauser, M. T., Dorbala, S., Di Carli, M. F., Fish, M. B., Ruddy, T. D., Bateman, T. M., Einstein, A. J., Kaufmann, P. A., Miller, E. J., Sinusas, A. J., Acampa, W., Han, D., Dey, D., Berman, D. S., & Slomka, P. J. (2024). Clinical phenotypes among patients with normal cardiac perfusion using unsupervised learning: A retrospective observational study. EBioMedicine, 99, 104930. https://doi.org/10.1016/j.ebiom.2023.104930
View source version on businesswire.com: https://www.businesswire.com/news/home/20240927874133/en/
GE HealthCare Media Contact:
Emmy Elguizaoui
+1 (978) 243-7503
Emmy.Elguizaoui@gehealthcare.com
Source:
